MLD is the sole owner of patents for measuring PC-594 and other gastric tract acids as a risk factor for pancreatic, colorectal and other cancers.
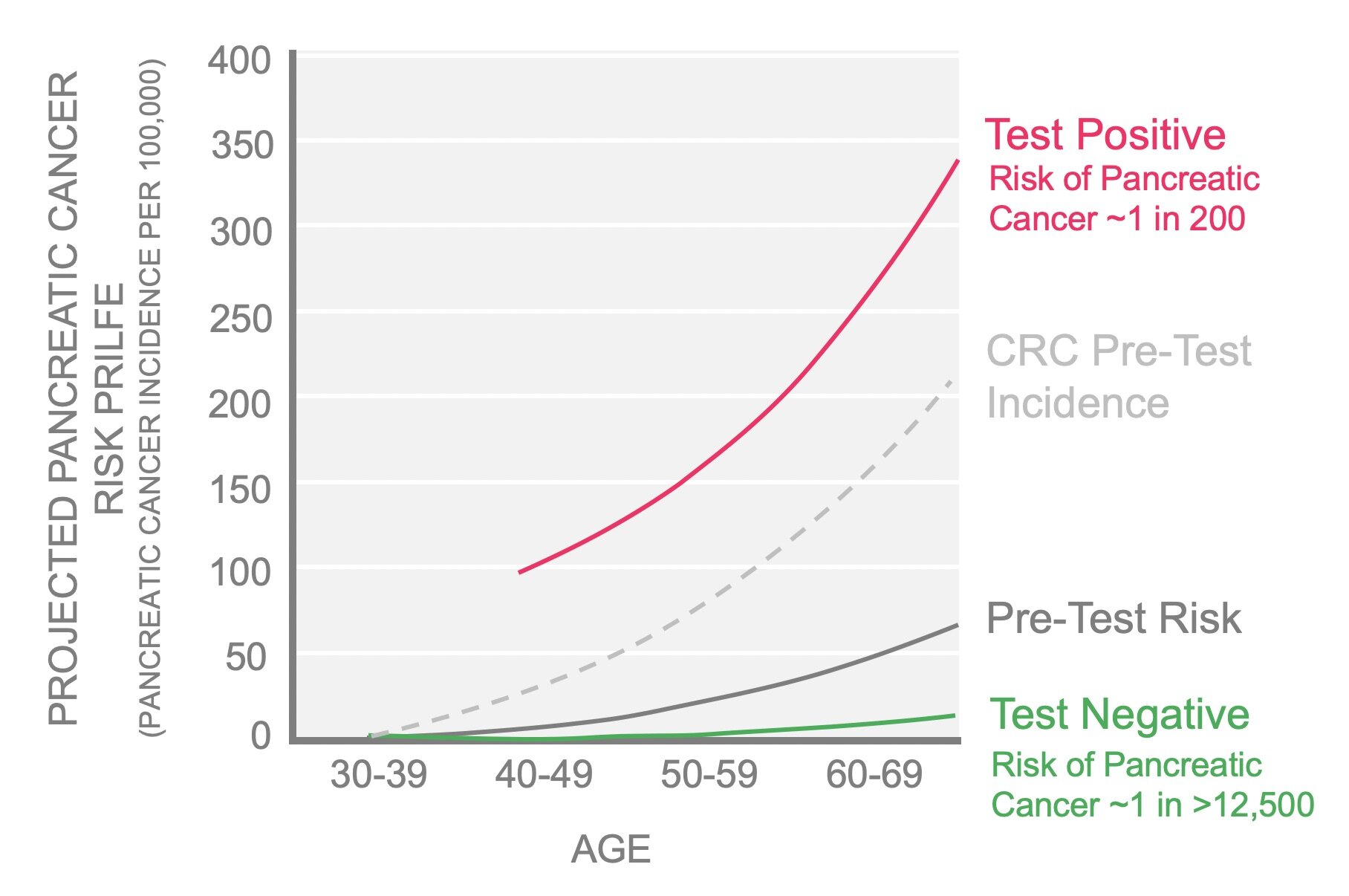
The PC-594 Test identifies increased risk by splitting the general population into two groups: one with a higher than average incidence of pancreatic cancer (pink line) and one with a lower than average incidence (green line). The solid grey line represents the pre-test current pancreatic cancer incidence by age in Canada. For any subject under age 65 who tests positive in the PC-594 Test, the risk of pancreatic cancer is up to four times greater than the risk associated with having three first degree relatives diagnosed with the disease.
For comparison, the incidence of colorectal cancer is plotted (dotted grey line). The incidence of colorectal cancer (CRC) beginning at age 50 (in the absence of other risk factors) is considered high enough to warrant the costs and risks of performing colonoscopy on everyone age 50 or older. Since the incidence of pancreatic cancer among subjects with positive PC-594 Test results is predicted to be at least 2.5 times higher than colon cancer in the general population (considered sufficient to warrant colonoscopy), assessing the population for pancreatic cancer risk with PC-594 based screening warrants further consideration.
The PC-594 Test Kit is a blood test that identifies people with an elevated risk of pancreatic cancer. Although pancreatic cancer has a high mortality rate, the incidence in the general population is too low to justify routine screening. The PC-594 Test changes this. The incidence of pancreatic cancer in subjects with positive PC-594 Test is up to 100-fold higher than in people with a negative result.
The PC-594 Test Kit is designed to be run on any triple-quadruple tandem mass spectrometer. The test measures levels of the metabolite PC-594 in the blood. If PC-594 levels are low (a positive test), risk of pancreatic cancer is high. A low PC-594 level doesn’t mean positivity for pancreatic cancer – rather it means that risk of pancreatic cancer is increased and further medical advice should be sought.
Subjects with a positive PC-594 test result should speak to their physician about managing their risk. This could include medical imaging or further surveillance.
The PC-594 test kit is intended for use as a research tool in risk assessment and monitoring; it is not a standalone diagnostic test, and is not a screening test for pancreatic cancer.
References
Serum metabolite profiling for the detection of pancreatic cancer. Results of a large independent validation study. Pancreas, August 11, 2016.
Pancreatic cancer serum biomarker PC-594: Diagnostic performance and comparison to CA19-9. World J Gastroenterology. 2015 Jun 7; 21(21): 6604–6612.
Metabolic system alterations in pancreatic cancer patient serum: potential for early detection. BMC Cancer 2013, 13, 416.