Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens
Rubio JM, Astudillo AM, Casas J, Balboa MA, and Balsinde J. (2018). Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens. Frontiers of Immunology, 9, 1723.
Photo by Dr_Microbe/iStock / Getty Images
If an injury or pathogen is detected by an organism, specialized cells called macrophages initiate an inflammatory response to activate other components of the immune system and perform phagocytosis, a process to rid the body of the damaged cell or pathogen. During phagocytosis, macrophages must reorganize their plasma membranes in order to engulf and digest apoptotic cells or invading pathogens. Macrophage plasma membranes contain receptors that are localized to lipid rafts, areas of the membrane that are rich in cholesterol and are used to recognize the infectious agents. In addition to phagocytosis, lipid rafts have an important role in membrane sorting and trafficking. One significant component of both membrane structure and lipid rafts are plasmalogens, a class of phospholipids that contain a vinyl-ether bond.
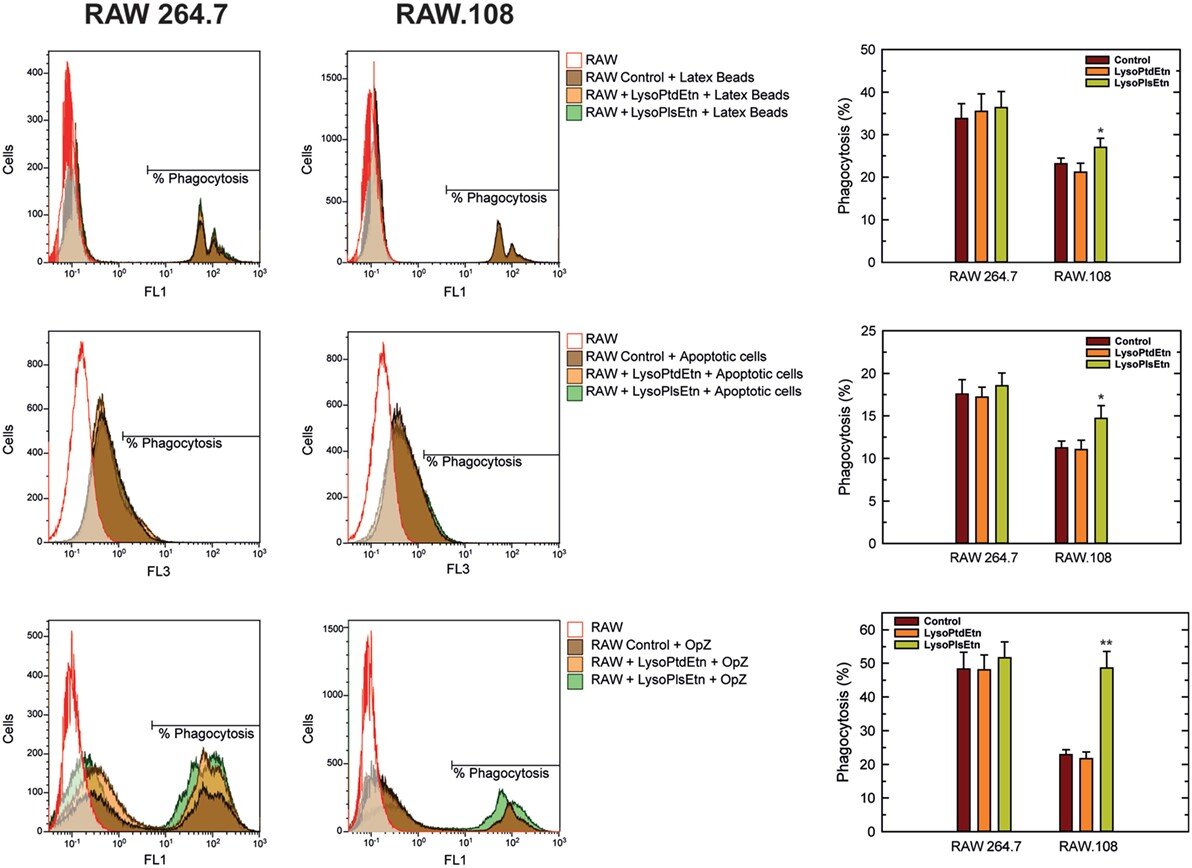
The role of plasmalogens on the membrane fluidity and phagocytosis of macrophages was recently investigated by Rubio et al who evaluated whether treatment with lysoplasmalogens (lysoPlsEtn) or lysophosphatidylethanolamine (lysoPtdEtn) resulted in incorporation of the lipids into plasmalogen-deficient macrophages and whether this recovered the observed phagocytosis deficiencies. Both the wild-type, RAW 264.7, and plasmalogen-deficient macrophage-like cell lines, RAW.108, were cultured, phagocytosis initiated, and the cells fixed and stained to determine the phagocytic index (number of phagosomes divided by the total number of cells in the field, multiplied by the percentage of phagocytosing cells). Phagocytosis was shown to be impaired in deficient cells, but treatment with lysoPlsEtn resulted in an increase in plasmalogen levels and an improved ability to phagocytize, seen in the figure below. This finding may indicate that rescuing the lipid deficiency improved the structure of the plasma membrane, enabling it to reorganize and to phagocytize. In addition, untreated plasmalogen-deficient cells showed greater membrane fluidity compared to wild-type macrophages, but when treated with lysoPlsEtn, this was reduced and normalized. The vinyl-ether bond in plasmalogens causes the sn-1 and sn-2 fatty acid side chains to adopt a more parallel confirmation and thus have a more compact structure. The results from this study indicate that increasing lipid species with this type of bond was able to normalize membrane fluidity to that of wild-type cells.
As a large component of lipid rafts are plasmalogens, Rubio et al also visualized rafts to determine if there were changes in the plasmalogen-deficient cells and if these changes were correlated with the cell’s ability to phagocytize. In resting and phagocytizing cells, treatment with lysoPlsEtn increased the number and size of the rafts in the wild-type and plasmalogen-deficient cells to above the levels seen in control treated wild-type cells. Wild-type cells and plasmalogen-deficient cells displayed a two-fold increase in lipid raft area per cell from about 6.5 um2 to 13 um2 and 4 um2 to 8 um2, respectively. The number of lipid rafts also increased from 10 to 15 per cell in the wild-type and from 8 to 13 rafts in the deficient line. An increase in lipid raft size and number would also increase the area containing receptors required for pathogen recognition and membrane sorting, which explains its association with the recovered ability to phagocytize.
Interestingly, treatment with lysoPtdEtn was unable to rescue any of the phenotypes described. Both treatments used in this study are ethanolamine phospholipids, but lysoPlsEtn contains a vinyl-ether bond and lysoPtdEtn contains an acyl bond at the sn-1 position. The fact that only lysoPlsEtn was able to improve the effects of plasmalogen deficiency and not diacyl-containing lysophospholipids signifies that the vinyl-ether structure is necessary for the observed phagocytic improvement. It is also important to note that the body cannot convert an acyl bond to a vinyl-ether bond, so supplementing with only lysoPtdEtn would not contribute to plasmalogen levels. These findings by Rubio et al again demonstrate the importance of plasmalogens in mediating membrane fluidity and lipid rafts, and the resulting consequences on a fundamental inflammatory process. The immune system is known to deteriorate with age, and this affects many macrophage processes including phagocytosis. As reduced plasmalogen levels are also seen with age and are associated with defective macrophages, treatment with plasmalogens could be a method to recover macrophage function and improve the ability to remove pathogens from the body.
Effect of lysoPlsEtn on the phagocytosis of different targets by RAW 264.7 and plasmalogen-deficient RAW.108 cells. The cells were either untreated or treated with 10 µM lysoPlsEtn or 10 µM lysoPtdEtn for 10 min as indicated. Afterward, unopsonized latex beads (5–7 particles/cell, 30-min incubation) or apoptotic Jurkat cells (ratio 3:1, 2-h incubation) were added, and phagocytosis was analyzed by flow cytometry. Measurements run in parallel using opsonized zymosan particles (OpZ, 5–7 particles/cell) are also shown for direct comparison. *Significantly different (p < 0.05) from untreated cells; **significantly different (p < 0.01) from untreated cells.